I
was thinking about submitting this for a pioneer award but upon learning of the
near impossible odds and apparent requirement for gravitas, which I don’t have
enough of, I decided against it. I put
it on the blog to lay out the idea and solicit comment.
Establishing efficacy of new neuroprotective therapies in neurocritical care and stroke has proven to
be an exercise in futility. Over 475 completed clinical trials are listed on the
Internet Stroke Trials Registry[1] with few apparent reproducible
results of any demonstrable efficacy in the acute context. However, these many negative studies belie
the supportive basic laboratory studies that justified the time and enormous
expense for such translational clinical trials. I will provide a rationale to suggest that such results, in retrospect,
are altogether predictable, suggest an explanatory model for such reproducible
futility in a complex biological system, and propose a research program to develop a multifaceted
approach which, taken altogether will produce breakthrough level data which
will then be generalizable to for multi-institutional application or study. Donnan [2] in the 2007 Feinberg lecture
suggests: “We have reached a stage at which research in this area should stop
altogether or radical new approaches adopted." The proposal is an answer to his plea for a
new approach.
I. Impact of perturbations in complex systems.
Single facet futility---multifacet therapeutic breakthrough
Imagine a factory that makes widgets. A number of processes are important for the
quality of the final widget as it proceeds: conveyor speed(x1), presence of raw
materials and power(x2), quality of
bolts(x3), quality of steel(x4), and
type of metal used for circuits(x5). A
weighting factor can be applied to each variable wi leading to the following general equation describing the widget
quality:
Q=w1x1+w2x2+w3x3+w4x4
Each variable x can be precisely known with very small
variation so any change in any of the variables will produce a reproducible and
predictable change in the widget quality Q.
In a biological system characterized by severity of a
pathophysiologically complex injury, S, a similar equation can be derived with
important pathophysiologic factors, xi,
and weighting factors, wi:
S=w1x1+w2x2+w3x3+w4x4……
Notably different from the widget however is that there are a
large number of disparate and potentially interacting factors known to
contribute to S with also an unknown number of as yet unknown factors with
correspondingly unknown weighting factors and variability. Moreover, each pathophysiologic factor xi has to be described over a
biologically diverse population such that each factor has an associated central
tendency and large normal or non-normal distribution about that mean. Additionally, in the context of clinical
medicine there are also associated system factors, Hi, like nursing ratio, nursing experience, availability of drugs
and technology, efficiency of rapid response teams, and so on, which are also
important to the severity of injury such that the equation can be written as
S=∑WiXi
+ ∑WiHi
Given the above characterization of the multiple highly
variable biological and system factors that enter into a given outcome, it
should come as no surprise that clinical studies directed at improving only one
of the above noted numerous complex factors tend to show no effect, especially
if multi-institutional in design (increasing variation in H factors), unless it
is truly a breakthrough phenomena (large W factor like early thrombolysis in
ischemic stroke) or the therapy exerts a multifaceted effect (e.g.,
hypothermia). This then leads to the
notion that the current widely accepted methods of advancing clinical knowledge
for complex problems is generally a fruitless waste of public resources which
produces innovation paralysis on the
part of institutions, third party
payers, clinicians, pharma, and
investigators, and that an alternate method is needed which is based on a
multifactorial approach. Rogalewski et al [3] have recently reviewed and endorsed
this concept, however, they fail to suggest a rational means for building the
multimodal approach other than trying everything at once…another prescription
for trouble. A rational method is
needed.
II. PDSA (Plan-Do-Study-Act) Cycles -- From QI
To Generalizability
A process designed to produce local improvement in the
quality of care (QI), the so-called PDSA
method advocated by Berwick [4] could provide a means to develop an
incrementally implemented multifactorial approach as a means to serially test and add single clinically
unproven but safe, pathophysiologically sensible and scientifically supported facets
in the therapy of a disease…eventually producing a multi faceted approach which
would then be amenable to more widespread testing and/or application.
The PDSA
method entails application of a nonrandomized process, using institutional and
individual (paired or N of 1) historical control data, to introduce incremental
improvements in processes of care. The
first step, planning, entails identification of a process to improve
with a plan for implementation. The do
phase entails the actual systematic implementation of a new process of
care. Studying then is the
procedure for collecting and analyzing the results of the new
intervention. And the act phase
provides for coming to a conclusion that the results are worthy or not worthy
of then producing a permanent change in a system’s health care protocols.
The PDSA
cycle is designed for processes of therapeutic QI which are meant to improve process-of-care
outcomes locally and not necessarily produce generalizable knowledge. Thus by the federal definition such processes
technically are not research. Nonetheless the PDSA process is a widely accepted type of institutional
process-of-care experimentation which very well could be a method to produce or
contribute to the production of new generalizable knowledge.
III. Using a single NICU with PDSA cycles to develop a multifaceted program
to arrest secondary brain injury.
A busy NeuroICU admits patients with traumatic brain injury,
intracerebral hemorrhage, subarachnoid hemorrhage, subdural hemorrhage, ischemic
stroke, and spinal cord injury. Each of
these diseases is associated with a
variety of similar secondary injury processes
which each individually are suitable targets for therapeutic intervention. One example of the widespread, albeit
incomplete, nature of these factors is illustrated in figure 1. Each of these
factors is associated with nucleotide polymorphisms such that a significant
variation between individuals can be expected in the response of any given
factor in the response to the initial insults, production of secondary injury,
and response to focused therapeutic interventions. Thus, as suggested by the equations modeled
in section I, these large variations in each factor makes it even more
difficult to identify a therapeutic effect. Moreover, treatment of one factor may be associated with exacerbation of
other factors. For example initial
efforts to identify calcium antagonists in the treatment of vasospasm were
fraught with problems with hypotension, which acted to exacerbate the ischemic process.

These notions lead to the proposed concept to incrementally
introduce therapeutic measures, each individually directed against single
facets in known processes of secondary injury after a neurologic insult, followed
by three primary measures:
1. Impact on the target pathophysiologic pathway
2. Surrogate neurologic outcome
measures
3. Functional neurologic measures.
Notably, chosen pathophysiologic goals should be widely
accepted to have neuroprotective potential based on either laboratory animal
studies or clinical studies. Modalities that prevent extremes of CBF,
anaerobic metabolism, glutamate toxicity hyper-metabolsim, and peroxidation are
examples of accepted pathophysiologic goals. Providing a therapy that produces such a goal will then be followed by
surrogate measures of neurologic effect along with subsequent measures of clinically
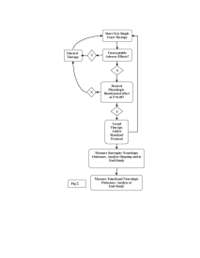
meaningful outcome. The decision flow
diagram is illustrated in figure 2.
Some examples of PDSA cycles with concomitant
pathophysiologic goals that can be envisioned are described below:
· Adjust blood pressure and paCO2 to optimize
CBF. Over the first five days after an
insult using continuous measurement technology and portable XeCTCBF optimize
CBF aided by measures of ICP, pbO2, EEG, and MD L/P.
· Administer antioxidant drugs. Measure urinary isoprostanes at baseline and after
implementation of therapy to confirm an antioxidant effect.
· Administer magnesium infusions to
control shivering. Evaluate effects on serum magnesium
levels, shivering index, temperature, and MD Mg++, glutamate and glycerol levels
· Administer reserpine as an
endogenous sympatholytic. Evaluate impact of this therapy
on MD catecholamine levels.
Abbreviations: CBF-cerebral
blood flow, MD-microdialysis, L/P
lactate pyruvate ratio, pbO2- pO2 of brain tissue, ICP-intracranial pressure, EEG- continuous electroencephalography,
CT-
and (c) MR-based infarct morphometry. Clinically meaningful functional outcomes are
evaluated one and six months after admission and both surrogate and functional
outcomes are statistically evaluated for a multifaceted therapy effect over the
course of the QI project.
By the end of the project multiple new neuroprotective therapies (vs
secondary injury)will be employed for
several clinical problems with concomitant QI measures for surrogate and actual
neurologic outcomes at baseline versus the end of the project as part of an IRB
overseen PDSA process. A robust difference in surrogate and actual
outcomes should be apparent. These results
will then form the basis for a rationally developed multifaceted approach to
prevention of secondary injury for multiple neurocritical care diseases with
robust effects apparent due to the multimodal approach. These
resultscan then be offered in aggregate as multifaceted therapies
ready for multi institutional trials or…for a true breakthrough effect…
possibly for immediate generalized implementation. Indeed this might be a new alternate model
for creation of new knowledge for complex medical problems.
1. Stroke
Trials Registry. The Internet Stroke Center 2008 January 4, 2024 [cited January 7, 2024]; Available from: http://www.strokecenter.org/trials/index.aspx.
2. Donnan, G.A., The 2007 Feinberg lecture: a new road map
for neuroprotection. Stroke, 2008. 39(1):
p. 242.
3. Rogalewski, A., et al.,
Toward a multimodal neuroprotective
treatment of stroke. Stroke, 2006. 37(4):
p. 1129-36.
4. Berwick, D.M., Developing and testing changes in delivery
of care. Ann Intern Med, 1998. 128(8):
p. 651-6.
5. Siman, R., et al., Novel surrogate markers for acute brain
damage: cerebrospinal fluid levels corrrelate with severity of ischemic
neurodegeneration in the rat. J Cereb Blood Flow Metab, 2005. 25(11): p. 1433-44.
 Mitch Keamy is an anesthesiologist in Las Vegas Nevada
Mitch Keamy is an anesthesiologist in Las Vegas Nevada
 Andy Kofke is a Professor of Neuro-anesthesiology and Critical Care at the University of Pennslvania
Andy Kofke is a Professor of Neuro-anesthesiology and Critical Care at the University of Pennslvania
 Mike O'Connor is Professor of Anesthesiology and Critical Care at the University of Chicago
Mike O'Connor is Professor of Anesthesiology and Critical Care at the University of Chicago
 Rob Dean is a cardiac anesthesiologist in Grand Rapids Michigan, with extensive experience in O.R. administration.
Rob Dean is a cardiac anesthesiologist in Grand Rapids Michigan, with extensive experience in O.R. administration.